Docuvera: 145% ROI, 82% Content Reuse, and a Successful Acquisition
As the founding designer, I named the company, developed its visual identity, and designed the product from inception through launch. Docuvera is now a core workflow for Eli Lilly and Boehringer Ingelheim, and was acquired by Cormeo (a Bertelsmann Investments company) in 2025.
Project Summary
Role: Founding Designer (Principal UX Designer)
Company: Author-it Software (Docuvera spun off as an independent company)
Duration: 2014 to 2016 (for the entire, fully-robust, deployed product. This includes initial discovery and research).
Scope: End-to-end: product naming, brand identity, design system, user research, and full product design from zero to launch.
Key Metrics
145%
ROI reported by pharmaceutical customers
110%
Productivity gains
82%
Content reuse rate
Acquired
Cormeo (Bertelsmann Investments), May 2025
The Problem
Bringing a new drug to market costs roughly $1 billion. Documentation accounts for an estimated $250 million of that, driven by inefficient workflows, poor content reuse, and fragmented systems for storing, editing, reviewing, and approving regulatory documents.
Medical authors at pharmaceutical companies were creating and managing highly complex, regulated documents across departments and geographies. Their primary tool was Microsoft Word. Content was manually copied and pasted between documents, introducing errors, redundancy, and compliance risks. Reviews and approvals happened through email chains and disconnected systems. There was no single source of truth.
Author-it Software saw an opportunity to build a component-based authoring platform purpose-built for life sciences documentation. I joined as the founding designer to take it from concept to product.
Starting with the Name and Identity
Before I could design the product, it needed a name and an identity. The platform was being built within Author-it Software, but needed its own brand to stand out in the life sciences market.
Naming
I led ideation sessions with the product team and the VP of Product. We generated several dozen initial concepts, none of which resonated. I then crowdsourced the effort, which produced hundreds more. Still no winner. I brought in an external naming consultant who proposed additional options.
One name stood out unanimously: Docuvera.
DOCU suggests documents and hints at the product's nature. VERA comes from the Latin "veritas," meaning truth and truthfulness. For a platform built around regulatory accuracy, the name felt right.
Visual Identity
I wanted the logo to combine three metaphors that represented the product's core purpose: science, components, and writing. A six-carbon benzene ring resembles a cube in isometric projection, and cubes can be stacked to build larger systems, making it a natural metaphor for components. Combining this with a pen tip completed the mark.
Color and Design Principles
Before selecting colors or visual patterns, I used the eMAP framework (developed by Boatwright and Cagan at Carnegie Mellon) to identify the emotional dimensions most important to the product's users. Enterprise software users in regulated environments needed to feel secure handling critical data, confident navigating complex workflows, and in control of their tasks.
These emotional insights became design principles that guided the entire product experience: Confidence through simplicity and clarity, Security through transparency, Control through contextual empowerment, Consistency through familiar patterns, and Delight through effortless interactions.
The color palette combined blue (trust, reliability) with green (growth, freshness) into what the team called "Docuvera Teal," a color that blends warmth and professionalism appropriate for the life sciences audience.
Designing the Product
With the identity established, I turned to the product itself. The core challenge was translating deeply complex regulatory workflows into an interface that medical authors, reviewers, and approvers could use intuitively, often working on the same document simultaneously across departments.
The Editor
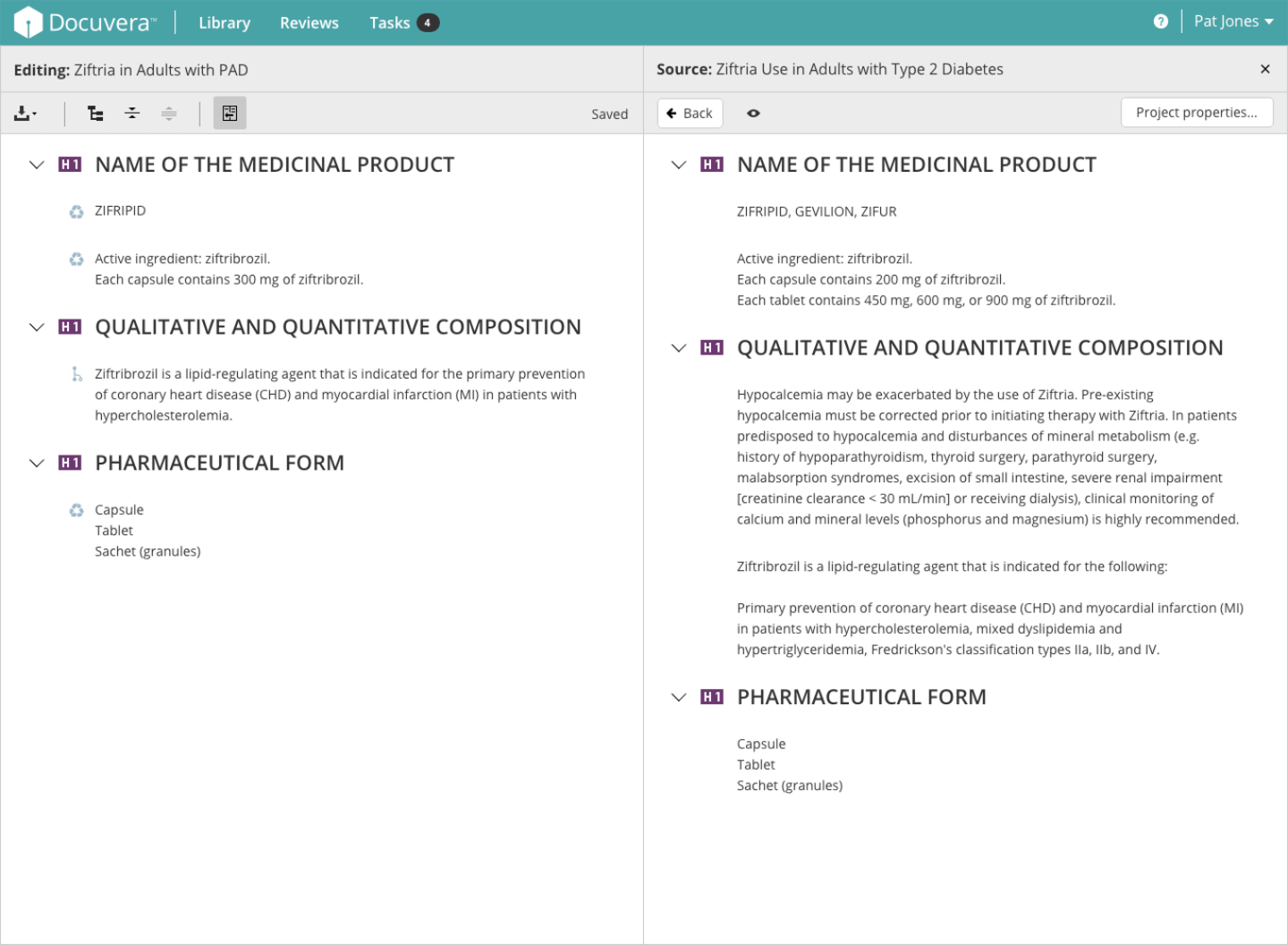
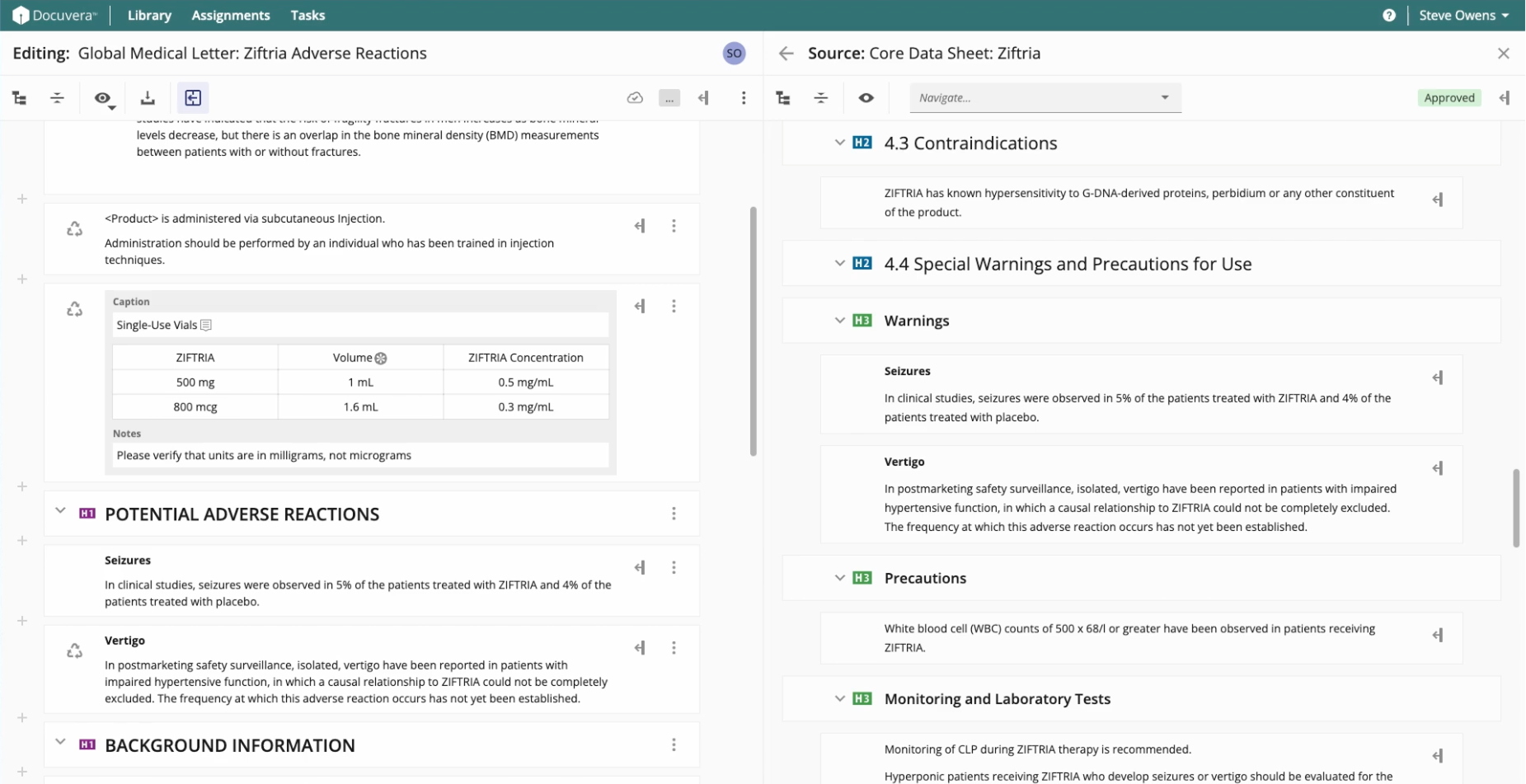
The editor is the core workspace where medical authors create and manage regulated documents. I designed it to support component-based authoring, meaning content is written in reusable blocks rather than monolithic documents. This was the key to achieving high content reuse rates. Authors can view the project structure alongside the document, compare the current document against other sources (such as a Core Data Sheet) in split view, and drag approved components directly into new documents.
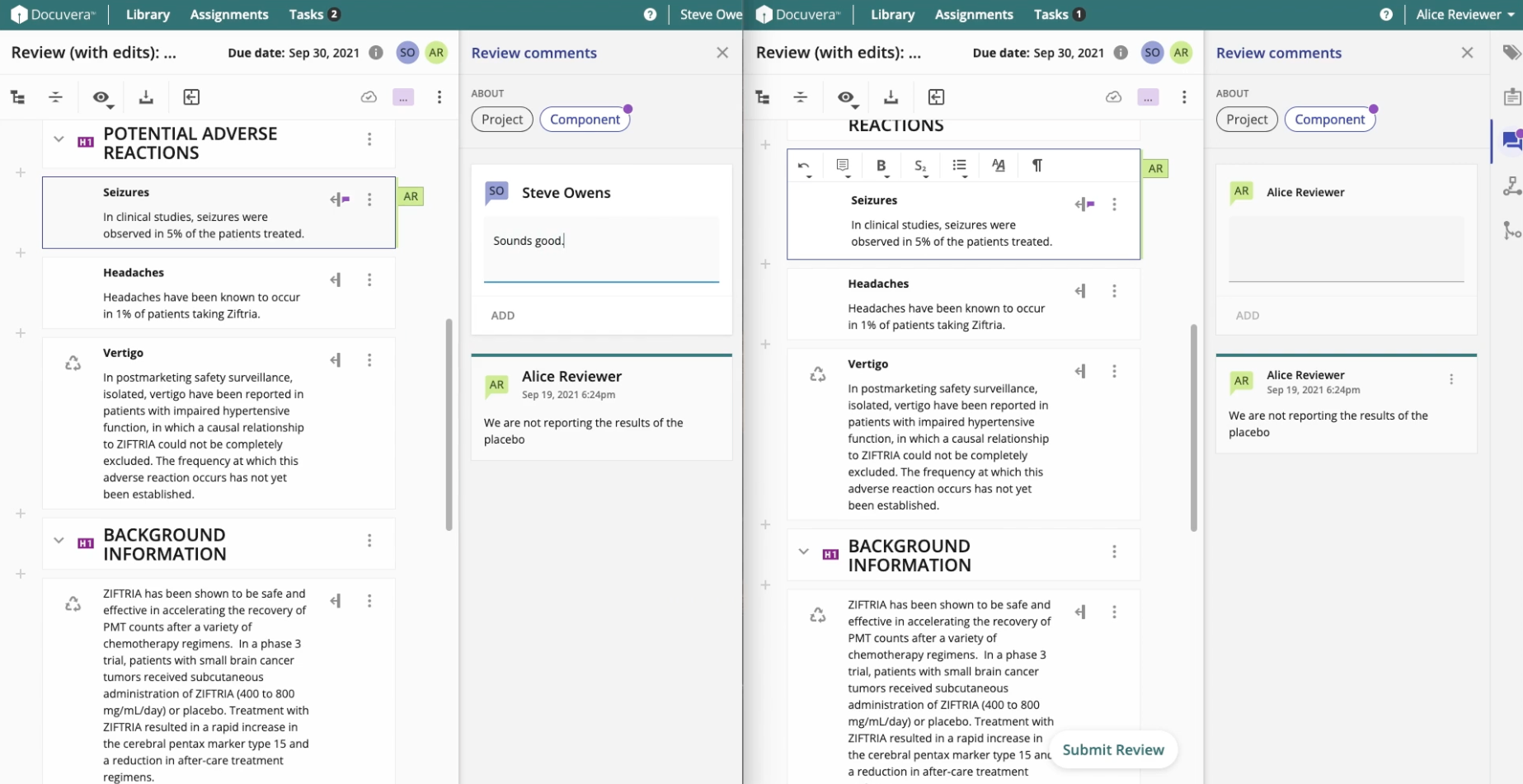
Collaboration
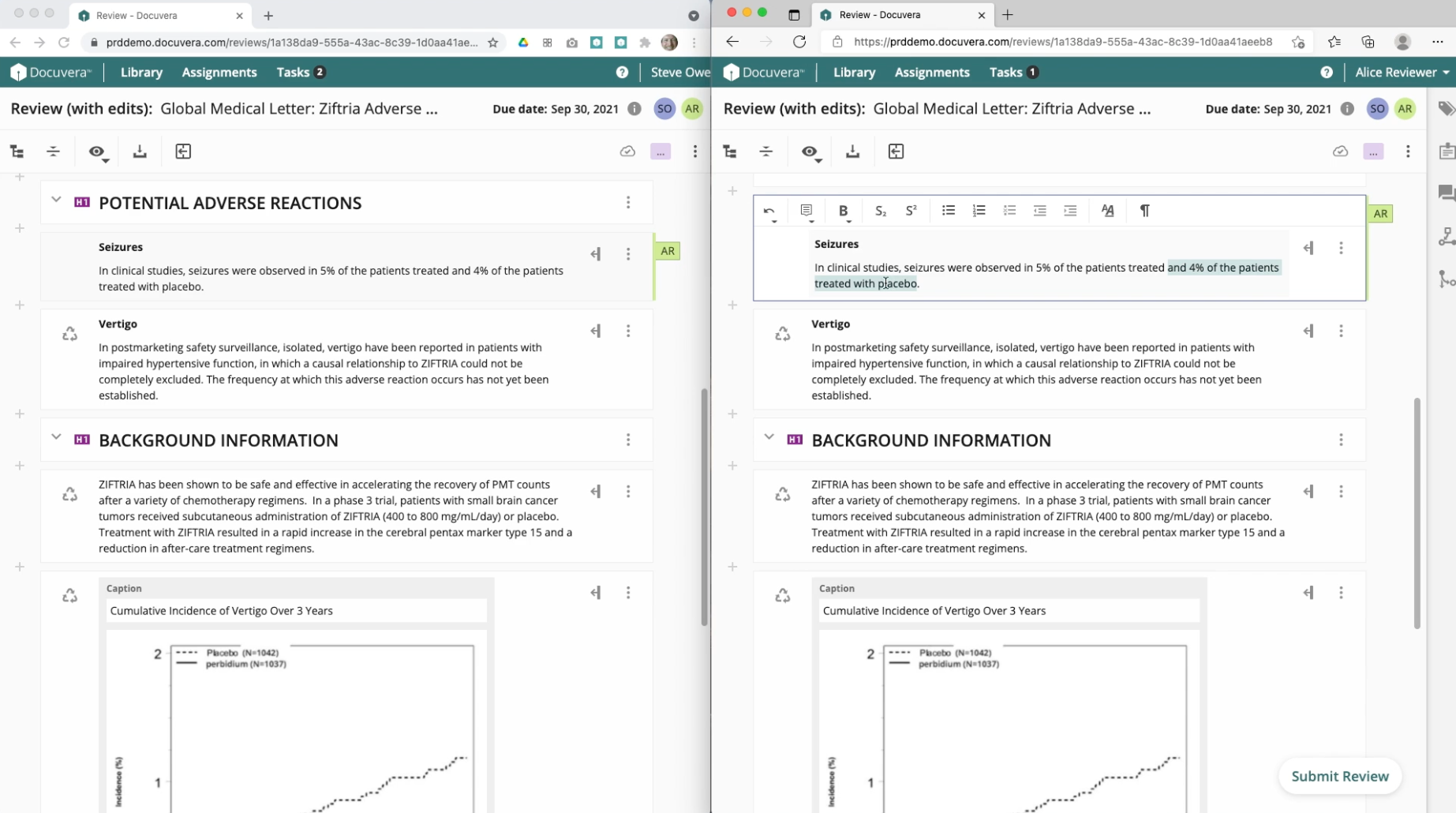
Pharmaceutical documents require input from multiple authors, reviewers, and approvers, often working simultaneously. I designed collaborative authoring to enable multiple users to edit different sections of the same document simultaneously, with clear visual indicators showing who is working where. A robust commenting system enables discussion at the component or project level.
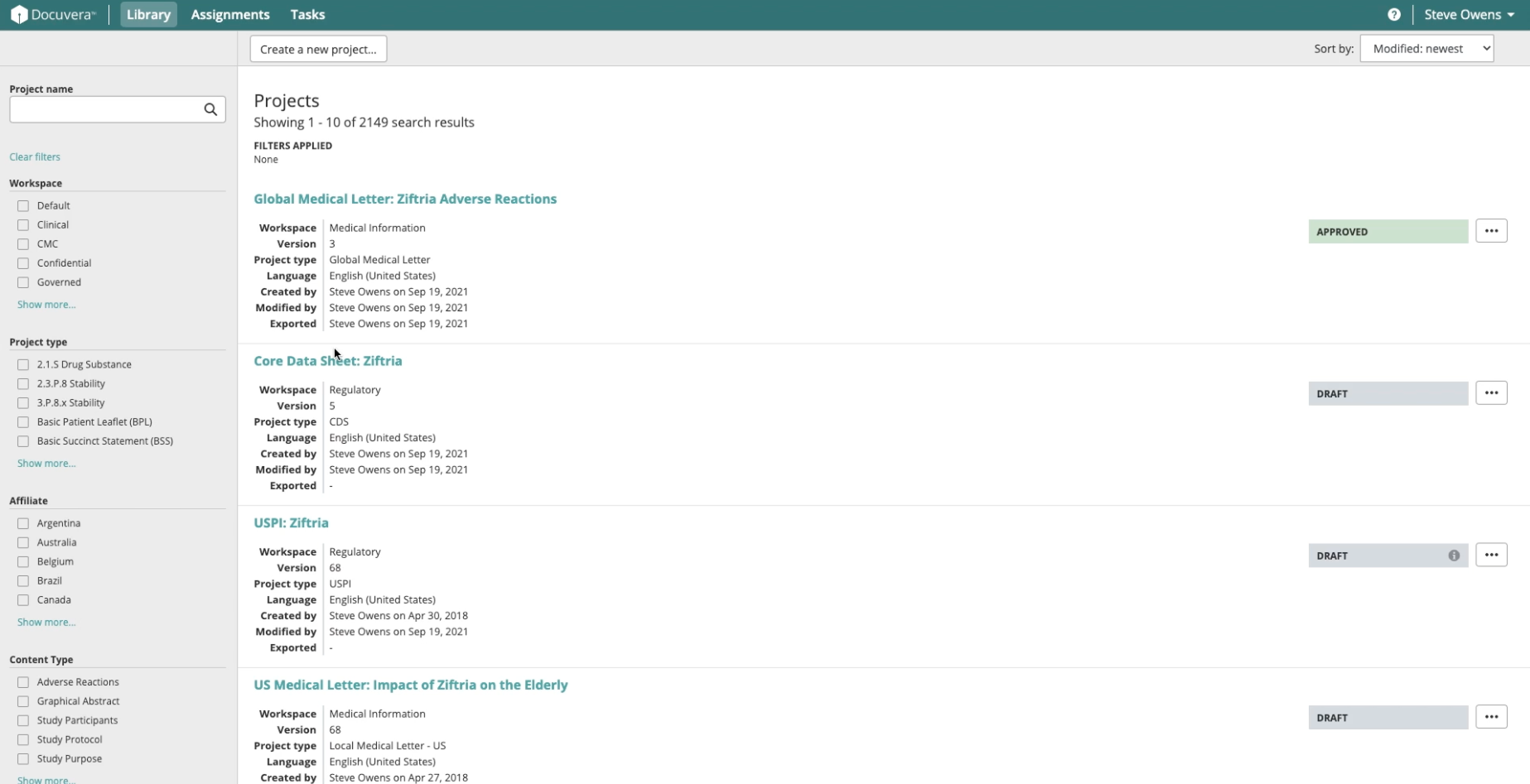
Library
Medical authors work across large volumes of regulated documents at various stages of completion. The Library view provides users with a clear view of each document's status (Draft, In Review, Approved) along with relevant metadata, making it easy to locate content and understand where things stand.
Reviews, Approvals, and Compliance
Medical authors work across large volumes of regulated documents at various stages of completion. The Library view provides users with a clear view of each document's status (Draft, In Review, Approved) along with relevant metadata, making it easy to locate content and understand where things stand.
Results
Over approximately three years as founding designer, I took Docuvera from concept to launched product, delivering the name, brand identity, design system, and the full application: editor, library, review and approval workflows, localization, translation, and admin experience.
The measurable impact reported by pharmaceutical customers:
145% return on investment. The platform paid for itself and then some, primarily through reduced documentation costs and faster regulatory cycles.
110% productivity gains. Authors using Docuvera were more than twice as productive compared to their previous workflows.
82% content reuse. Component-based authoring eliminated redundant content creation across documents, reducing errors and ensuring consistency.
Adopted by major pharmaceutical companies. Docuvera became a core workflow for Eli Lilly, Boehringer Ingelheim, and other life sciences organizations.
Spun off as an independent company. The product's success led Author-it to spin off Docuvera as a standalone entity. The company itself adopted the product name I created.
Acquired by Cormeo (Bertelsmann Investments) in May 2025. The ultimate validation of the product's market value.
Testimonial
“Working in product for the last 25 years, I have seldom come across a UX/UI person that could balance the mechanics of user experience design with the aesthetics of UI visual design. Vytas is the best person with whom I have worked to balance these skills.
For example, Vytas was responsible for defining the user interaction of a very complex, component authoring SaaS service where our core focus was to build an experience that was as easy as Microsoft Word. I am proud to say that our customers universally acknowledge the simplicity of our application and the rapid adoption of our enterprise application is a further testament to its ease of use.
In addition to the user experience, Vytas was also responsible for the look-and-feel of the application and built a design that reinforced simplicity, cleanliness, and lack-of-clutter.”
See Docuvera in Action
Steve Owens, CEO of Docuvera, walks through the product in this overview.